Either for orthopaedics or dental applications, biomaterials research has shown a huge expansion in the last decades. Indeed, first based on metal or ceramic or polymers, the research has gone to nanoparticles and “active” biomaterials.
“Materials scientists have been playing important roles in the design and manufacturing of orthopedic implants and tissue engineering scaffolds for several decades. However, until more recently, they have not had much involvement in cancer therapy” (source: The evolution of biomaterials research – Volume 16, Issue 11, Page 408–409 | Amir A. Zadpoor).
Atlantic Bone Screen can evaluate the efficacy and cytotoxicity of your biomaterials / medical device using an in-vitro model using L929 cells.
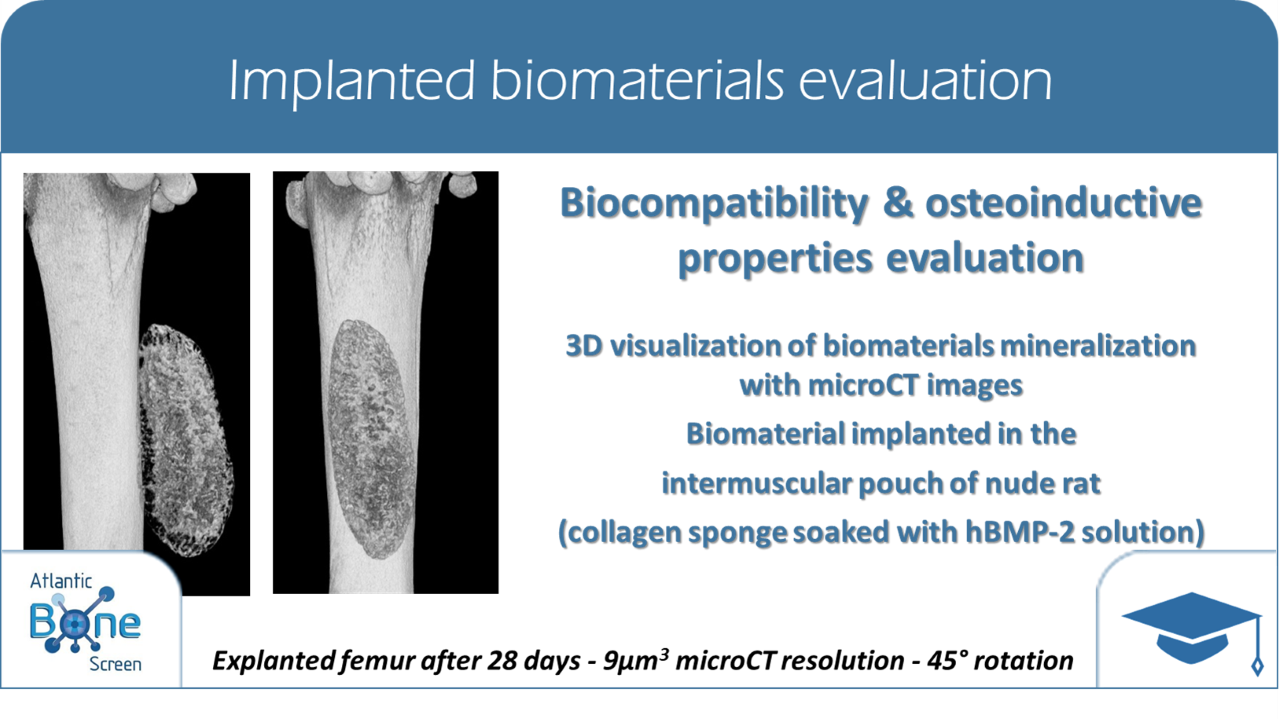
Atlantic Bone Screen also provides different models (implantations, time, animal model…) in order to help Biomaterials research and industries in the evaluation of the osteoinduction of their compound, its resorption, its ability to become a bone substitute for the orthopedics or dental markets.
The definition of the study design and the analyses are performed according to ISO 10993-5 (especially part 5 and part 6).
KEYWORDS
Bone reconstruction implantations, Muscular and subcutaneous implantation, Trabecular, cranial defects, Histopathology of biomaterials in situ, Evaluation of bone formation rate and osteointegration, Evaluation of bone mineralization, thrombosis and degradation, Osteoformation and bone substitute evaluation, Osteoinduction models
See our in-vivo models for biomaterials evaluation>
See our in-vitro models>
See our histology services for biomaterials evaluation >