Atlantic Bone Screen has the advantage of using its own animal facility to provide in-vivo assays to its customers. Biocompatibility of medical devices and therapeutic compounds can be evaluated within the scope of CE Labelling or Marketing Approval (MA).
Models are chosen and validated by our Scientific Team, our veterinary pathologist and with our expert consultants network. Either standard or tailor made models can be proposed for biocompatibility evaluation.
Atlantic Bone Screen provides non-GLP biocompatibility evaluation of therapeutic compounds (macroscopic and histopathological analyses) and biomaterial studies can be done on different species/models but always referring to ISO 10993-6.
- Single dose studies – Multidose studies – Biomaterials / Medical device implantation
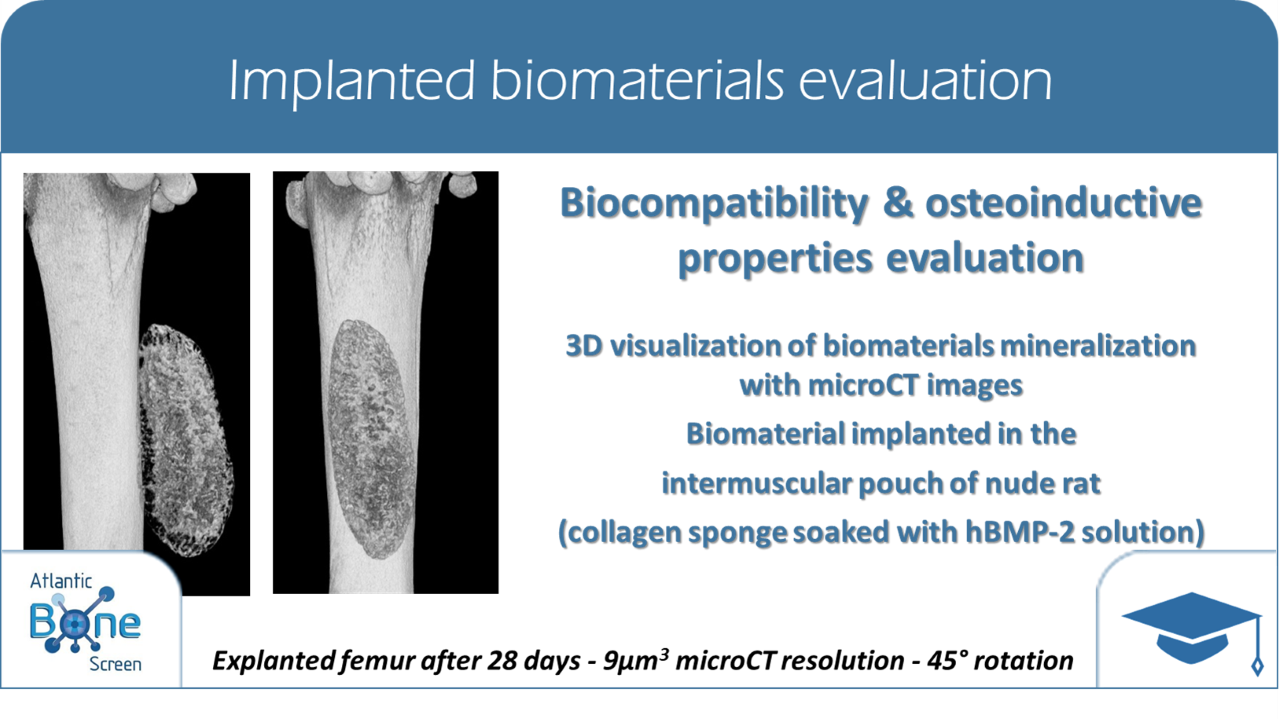
- Evaluation of biomaterials osteoinductive properties implanted into muscles or in subcutaneous implantation
- Evaluation of biomaterials-induced new bone formation after its implantation into bone defect
EXAMPLES OF TESTED BIOMATERIAL
- cement,
- gel,
- powder, granules
- membrane
- …
EXAMPLES OF BIOMATERIALS IMPLANTATIONS
- Sub-cutaneous implantation
- Muscular implantation
- Critical size defect in femoral condyle
- Critical size defect in parietal bone
Atlantic Bone Screen supports its Partners and Customers in the study design and choice of the implantation, study duration and time points depending on the biomaterial type, the goal of the study, the final application of the biomaterial, the available data from past investigations…
For these studies, Atlantic Bone Screen can rely on highly qualified scientist and technicians and on a large range of analyses techniques for biomarkers analysis, imaging analysis and histological services.